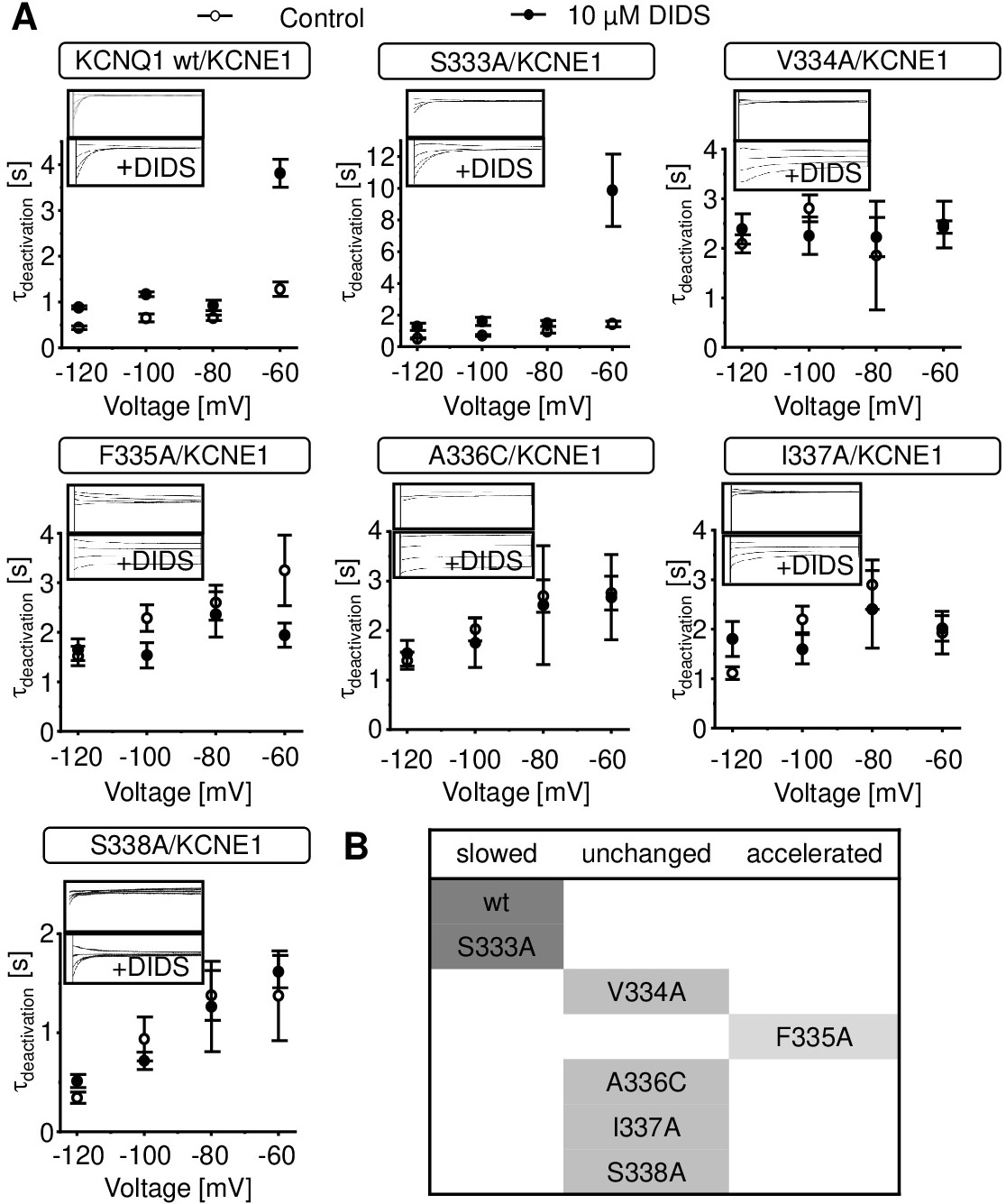
Fig. 5. Influence of KCNQ1 pore domain mutations on the DIDS-mediated slowdown of KCNQ1/KCNE1 channel deactivation. A. Effect of 10 ÁM DIDS on deactivation kinetics of wildtype and mutant KCNQ1/KCNE1 channels. τdeactivation was calculated by fitting deactivation traces to a single exponential function (Q1 wt n = 7-11; S333A n = 5-10; V334A n = 3-6; F335A n = 12-22; A336C n = 7-20; I337A n = 11-28; S338A n = 4-7, ▒ SEM). Representative currents are shown as small inlay representations. Currents were recorded using the deactivation protocol (3) described in methods section. B. Table of deactivation effects by 10 ÁM DIDS of wildtype and mutant KCNQ1/KCNE1 channels.